Disposable infusion set (TOTM infusion set)
key word:
Classification:
Infusion& transfusion sets
- Product Description
-
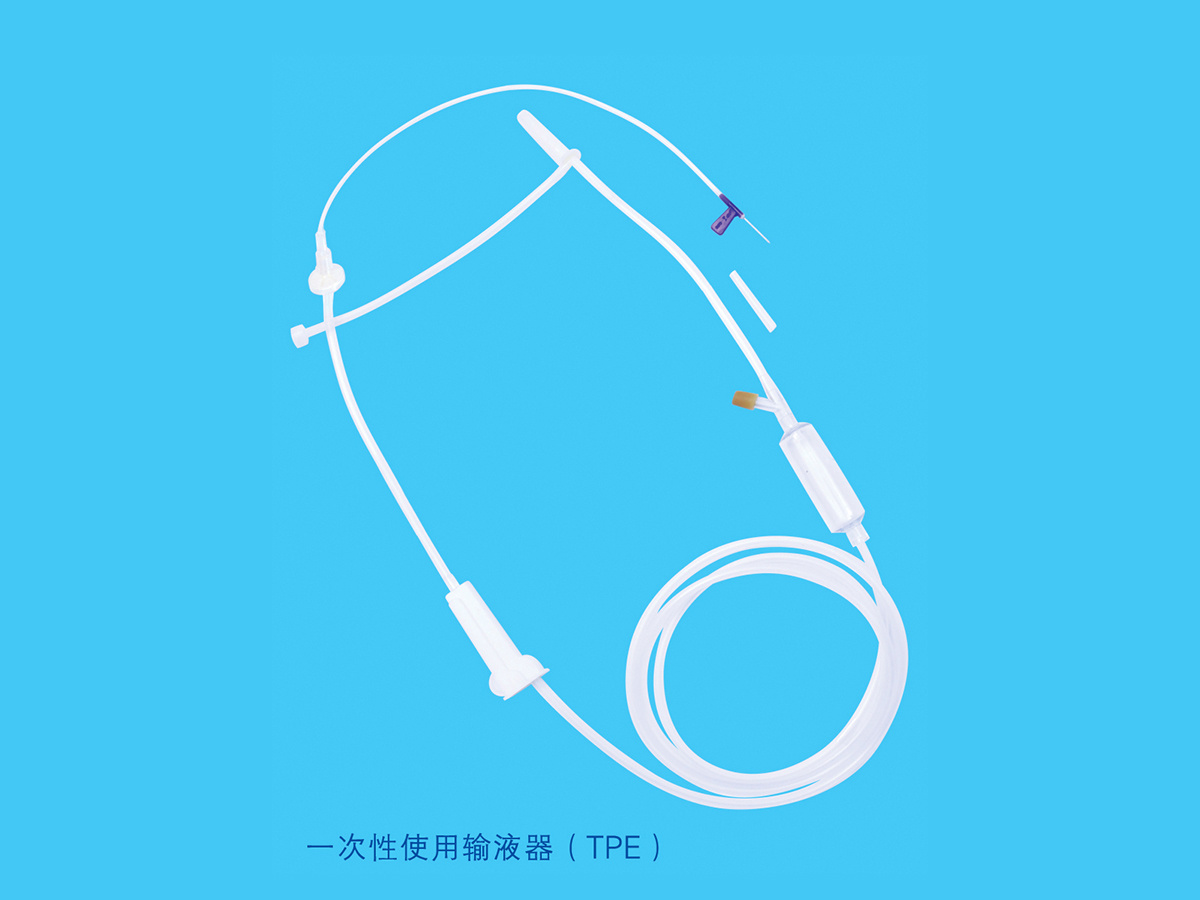
Product name: Disposable TOTM infusion set with needle
Model:H type, J type, K type
Needle specification: 0.45mm, 0.50mm, 0.55mm, 0.60mm, 0.70mm, 0.80mm, 0.90mm, 1.2mm
Scope of application: This product is used by medical units for intravenous infusion of liquid medicine.
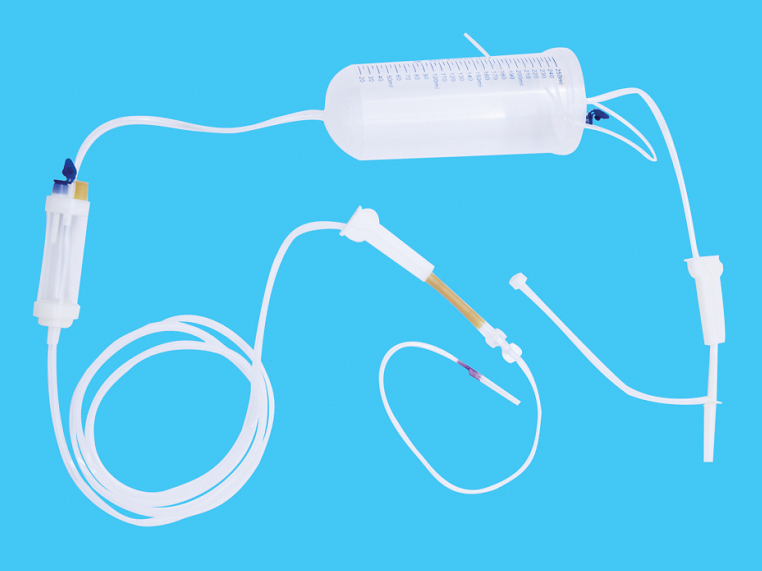
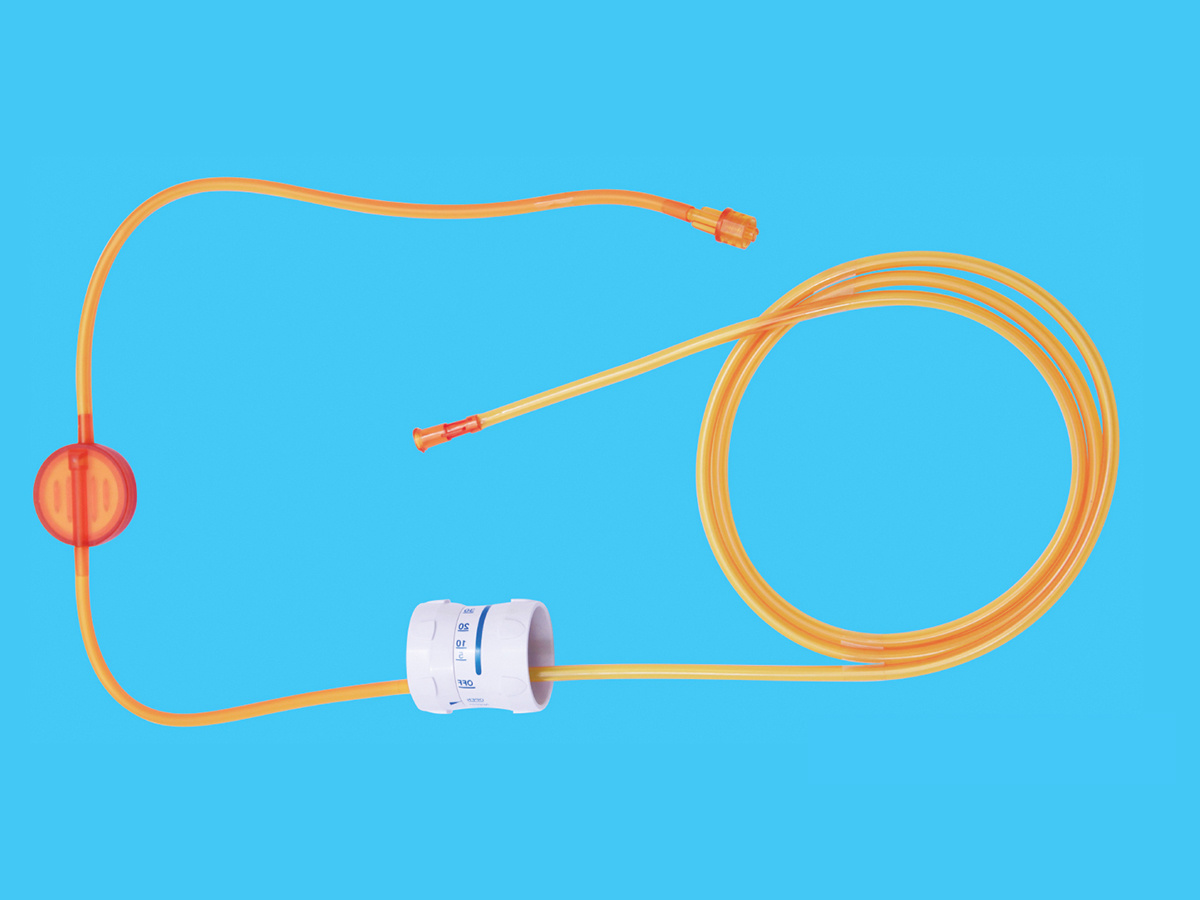
Product performance structure and composition: The disposable TOTM infusion set with needle product consists of a cork puncture protective cover, a cork puncture, an air filter, a pipeline, a dropper, a dropper, and a liquid medicine. Injection parts, flow regulator, common liquid filter (15µm), latex tube (for blood return only), medical infusion patch and disposable intravenous infusion needle are optional accessories. Water stop clips and disposable intravenous infusion needles are optional accessories. The product is sterilized by ethylene oxide for one-time use.
Product performance advantages:
1. This product does not contain o-benzene DEHP plasticizer;
2, this product uses TOTM plasticizer;
3. The TOTM infusion set has been tested for drug compatibility. According to the dissolution study, the clinical use of this product for children, adolescent men, pregnant and lactating women brings acceptable risks when infusing liquids. of.
Instructions for use:
Currently, traditional infusion sets are made of polyvinyl chloride (PVC) with diethyl phthalate (DEHP) plasticizer. In recent years, there have been reports that the PVC infusion set has an adsorption effect on certain drugs, which leads to a decrease in efficacy and that the precipitation of plasticizers during the infusion process poses risks to human health. The performance of DEHP in experimental animals has attracted wide attention from relevant departments at home and abroad. According to the REACH regulations, the US FDA and the European Union have issued public health warnings on PVC medical devices containing plasticizer DEHP "It is recommended that high-risk groups (such as infants and pregnant women) use DEHP alternatives". China's CMDE issued "one time" in 2010. The guiding principles for the registration and application of sex-use infusion devices” point out that“is no longer limited to DEHP, and safer medical plasticizers can be used”. Therefore, the development of alternative materials to eliminate the potential harm of DEHP plasticizers should be a new task for medical device researchers. TOTM, as a plasticizer instead of DEHP, is mainly to reduce the potential hazards of plasticizers. For PVC products of TOTM plasticizers, research on drug compatibility is carried out through experiments. A disposable infusion solution produced by TOTM plasticizers The risks brought by the infusion of liquid medicine to adults are all acceptable, especially for children, prepubescent men, pregnant and lactating women. The risks caused by liquid infusion are all acceptable. .
Obtain product quotation




 0791-85628888
0791-85628888 

 Email
Email 